Enasidenib is an oral isocitrate dehydrogenase-2 (IDH2) inhibitor, which was first approved for marketing in the United States in 2017 for the treatment of specific types of acute myeloid leukemia (AML). As a targeted therapy, Enasidenib inhibits the activity of mutated IDH2 enzyme, thereby reducing the level of 2-hydroxyglutarate (2-HG) in tumor cells, promoting the differentiation of leukemia cells and inhibiting their proliferation.
What Are the Indications for Enasidenib?
Indications
Enasidenib is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML), who must be confirmed to have IDH2 gene mutation by an FDA-approved detection method.
This indication is established based on the results of a single-arm, multicenter clinical trial. The primary efficacy endpoints include complete remission rate, duration of remission, and improvement in transfusion dependence.
Patient Selection
Genetic testing is a prerequisite: Before using Enasidenib, the presence of IDH2 mutation must be confirmed through blood or bone marrow sample testing. Currently FDA-approved companion diagnostic tests are available to guide medication use.
Applicable disease stage: It is only indicated for AML patients whose disease has relapsed after previous treatment or is refractory to treatment. It is not suitable for newly diagnosed AML or other types of leukemia.
Age restriction: The safety and efficacy of this drug in pediatric patients have not been established yet, so it is only for use in adults.
Specifications and Properties of Enasidenib
Specifications and Dosage Forms
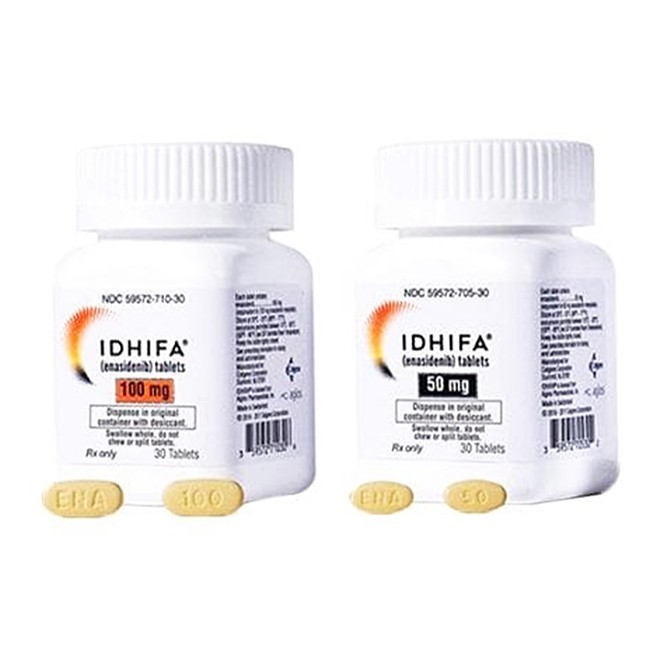
50 mg tablet: Each tablet contains 50 mg of Enasidenib (equivalent to 60 mg of Enasidenib mesylate). The tablet is pale yellow to yellow, oval-shaped, engraved with "ENA" on one side and "50" on the other.
100 mg tablet: Each tablet contains 100 mg of Enasidenib (equivalent to 120 mg of Enasidenib mesylate). The tablet is pale yellow to yellow, capsule-shaped, engraved with "ENA" on one side and "100" on the other.
Properties and Composition
Enasidenib has a complex chemical structure, and its mesylate salt has extremely low solubility in water.
In addition to the active ingredient, the tablet contains a variety of inactive excipients, including colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose acetate succinate, yellow iron oxide, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulfate, sodium starch glycolate, talc and titanium dioxide. These ingredients together ensure the stability and compressibility of the drug.
Storage Method for Enasidenib
Storage Environment Requirements
Temperature: Store at room temperature between 20°C and 25°C (68°F and 77°F), with short-term excursions permitted between 15°C and 30°C (59°F and 86°F).
Container: Always store in the original drug bottle, which contains a desiccant canister to absorb moisture.
Sealing: Tighten the bottle cap immediately after use to prevent the drug from being exposed to air.
Moistureproof and Safety Precautions
Enasidenib is relatively sensitive to moisture, and humid environments may affect the stability of the tablets.
Keep the original bottle packaging during storage and dispensing; do not transfer the tablets to other containers.
This product should be placed out of the reach of children to avoid accidental ingestion.
Special Disposal Reminder
Patients should be informed that unused drugs should not be discarded at will, but disposed of through standardized drug recycling channels to reduce potential risks to the environment and others.