Enasidenib is a targeted drug indicated for relapsed or refractory acute myeloid leukemia (AML) with IDH2 mutation, and its trade name is IDHIFA. Given its specific indications and prescription-only nature, patients and their family members need to understand the legitimate procurement channels, pay attention to medication safety, and learn to identify the authenticity of the drug.
What Are the Procurement Channels for Enasidenib?
Overseas Purchase
Patients can choose to consult and purchase the drug at hospital pharmacies or licensed pharmacies in countries or regions where Enasidenib has been approved for marketing.
As drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase via Medical Service Institutions
Patients can consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions can usually provide legal import channels as well as professional consultation and guidance.
Precautions for Purchasing Enasidenib
Strictly Follow the Doctor’s Advice
Enasidenib is a specialized targeted drug, and a prescription must be issued by a physician based on the results of genetic testing (IDH2 mutation).
Patients must not self-assess their condition or purchase the drug based on personal experience.
Confirm the Drug Version and Specification
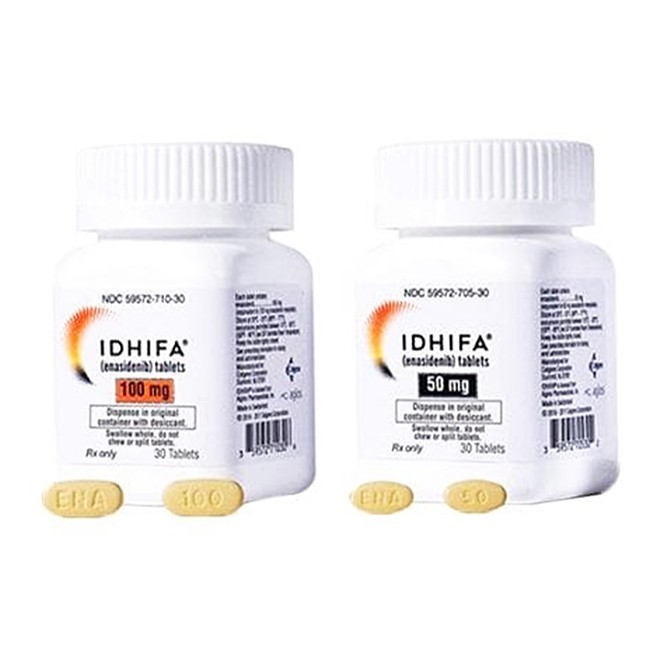
Enasidenib is currently available in two specifications: 50 mg and 100 mg.
Patients need to confirm the required dosage according to the doctor’s advice and check the markings on the tablets (e.g., “ENA50” or “ENA100”).
Understand the Drug Storage Conditions
Enasidenib should be stored at room temperature between 20°C and 25°C, protected from moisture.
Before purchasing, inspect the packaging for integrity, check whether a desiccant is included in the bottle, and ensure the drug is used within its expiration date.
Monitoring During Medication
Before medication initiation and in the early stage of treatment, regular monitoring of blood routine, liver and kidney function, and other indicators is required to prevent serious adverse reactions such as differentiation syndrome and tumor lysis syndrome.
Patients should maintain close communication with their doctors and report any discomfort symptoms promptly.
How to Identify the Authenticity of Enasidenib?
Inspect the Outer Packaging and Label
The packaging of genuine drugs should feature clear printing and complete information, including the drug name, specification, batch number, expiration date, manufacturer (e.g., Bristol Myers Squibb), and import drug registration certificate number.
The bottle should be properly sealed, with a desiccant enclosed.
Verify the Tablet Appearance
The 50 mg tablet is pale yellow to yellow, oval-shaped, engraved with “ENA” on one side and “50” on the other.
The 100 mg tablet is pale yellow to yellow, capsule-shaped, engraved with “ENA” on one side and “100” on the other.
If the tablet’s color, shape, or engraving is blurred or inconsistent with the above descriptions, the authenticity of the drug should be highly suspected.