Enasidenib is an oral targeted therapy drug, marketed under the brand name IDHIFA®, which was first approved for marketing in the United States in 2017. It is an isocitrate dehydrogenase-2 (IDH2) inhibitor, used for the treatment of specific types of Acute Myeloid Leukemia (AML).
What are the indications for Enasidenib?
Primary Indications
(1) Enasidenib is indicated for the treatment of adult patients with relapsed or refractory Acute Myeloid Leukemia (AML) with an IDH2 mutation, as detected by an FDA-approved test.
(2) This drug is not indicated for the treatment of AML patients in whom an IDH2 mutation has not been detected. Therefore, genetic testing of blood or bone marrow samples to confirm mutation status is mandatory prior to use.
Enasidenib Specifications and Characteristics
Drug Specifications
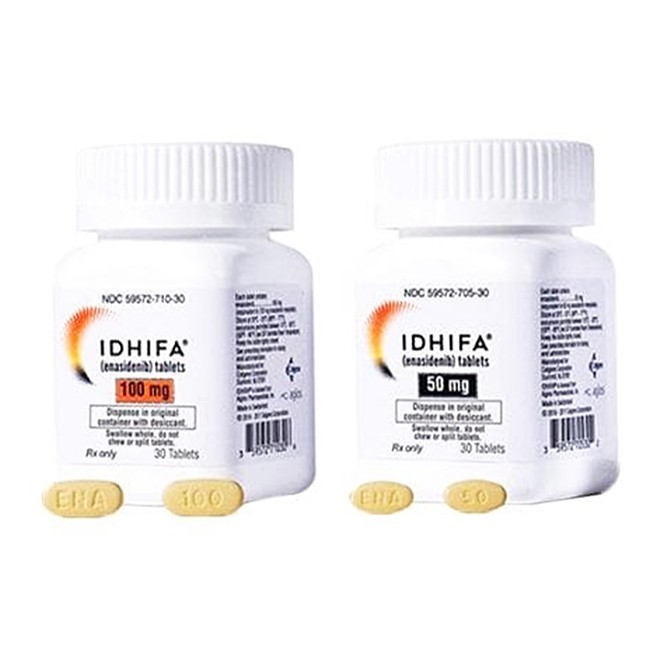
(1) 50 mg tablet: Each tablet contains 50 mg of enasidenib (equivalent to 60 mg of enasidenib mesylate).
(2) 100 mg tablet: Each tablet contains 100 mg of enasidenib (equivalent to 120 mg of enasidenib mesylate).
(3) Both strengths are supplied in bottles containing 30 tablets each, with a desiccant canister included.
Drug Characteristics
(1) 50 mg tablet: Appears as a pale yellow to yellow, oval, film-coated tablet debossed with "ENA" on one side and "50" on the other.
(2) 100 mg tablet: Appears as a pale yellow to yellow, capsule-shaped, film-coated tablet debossed with "ENA" on one side and "100" on the other.
Composition
(1) Both the 50 mg and 100 mg tablets contain the same inactive ingredients.
(2) These include: colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose acetate succinate, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulfate, sodium starch glycolate, talc, and titanium dioxide. These excipients collectively ensure the stability and compressibility of the drug.
Enasidenib Storage Method
Storage Conditions
(1) Enasidenib should be stored at room temperature, specifically between 20°C to 25°C (68°F to 77°F).
(2) Short-term excursions are permitted within the range of 15°C to 30°C (59°F to 86°F).
(3) The drug must always be kept in its original bottle with the cap tightly closed.
(4) The desiccant canister included in the bottle helps absorb moisture and prevent the tablets from deteriorating due to dampness; it should not be removed.
Special Storage Reminders
(1) Due to enasidenib's sensitivity to moisture, tablets should be taken immediately after removal from the bottle.
(2) Do not transfer tablets to other containers (e.g., weekly pill organizers) unless the container provides equivalent moisture protection and a tight seal.
(3) When the drug is no longer needed or has expired, it should be disposed of safely by following local regulations or through a drug take-back program. It should not be disposed of in household trash or wastewater.