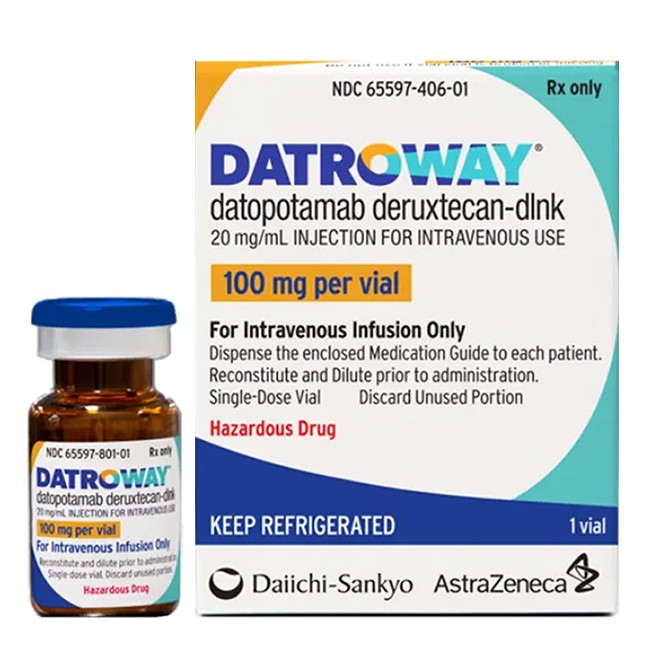
Datroway (datopotamab deruxtecan) is a Trop-2-targeted antibody-drug conjugate developed by Daiichi Sankyo Co., Ltd., which was approved by the U.S. FDA in 2025. It is indicated for the treatment of adult patients with unresectable or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer who have previously received endocrine therapy and chemotherapy.
Dosage and Administration of Datroway (Datopotamab Deruxtecan)
Route of Administration and Dosage Specifications
Datroway must be administered via intravenous infusion; intravenous bolus or rapid injection is strictly prohibited.
The drug is supplied as a 100 mg lyophilized powder in a single-dose vial.
The recommended dosage is 6 mg/kg (maximum dose of 540 mg for patients with body weight ≥ 90 kg), administered once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity occurs.
Premedication
Preservative-free lubricating eye drops: At least four times daily, with additional doses as needed.
Corticosteroid-containing mouthwash (0.1 mg/mL dexamethasone oral solution): Four times daily.
Antihistamine (diphenhydramine 25-50 mg): Administered intravenously or orally.
Antipyretic and analgesic (acetaminophen 650-1000 mg).
5-HT3 serotonin receptor antagonist or other appropriate antiemetic.
Infusion Regimen
First infusion: Duration of 90 minutes; monitor for at least 1 hour after infusion.
Second infusion: If the first infusion is well-tolerated, duration of 30 minutes; monitor for at least 1 hour after infusion.
Subsequent infusions: If the previous infusion is well-tolerated, duration of 30 minutes; monitor for at least 30 minutes after infusion.
Dosage Adjustments for Datroway (Datopotamab Deruxtecan)
Dosage Adjustments Based on Adverse Reactions
First dose reduction: 4 mg/kg (maximum 360 mg).
Second dose reduction: 3 mg/kg (maximum 270 mg).
If the patient cannot tolerate the 3 mg/kg dose, Datroway should be permanently discontinued.
Interstitial Lung Disease (ILD)/Pneumonitis
Asymptomatic (Grade 1): Suspend administration until complete resolution, then resume treatment.
Symptomatic (Grade 2 or higher): Permanently discontinue and immediately initiate corticosteroid therapy.
Ocular Adverse Reactions
Non-confluent superficial keratitis: Suspend administration until improvement.
Confluent superficial keratitis or corneal epithelial defect: Reduce by one dose level.
Medication in Special Populations for Datroway (Datopotamab Deruxtecan)
Reproductive-Age Population
Women of childbearing potential should undergo a pregnancy test before treatment initiation.
Effective contraception must be used during treatment and for 7 months after the last dose.
Male patients with female partners of childbearing potential should use effective contraception during treatment and for 4 months after the last dose.
Pregnancy and Lactation
Based on the mechanism of action, Datroway may cause fetal harm.
Breastfeeding is prohibited during treatment and for 1 month after the last dose.
Patients with Hepatic or Renal Impairment
Mild renal impairment (CLcr 30-89 mL/min): No dosage adjustment is required, but close monitoring of adverse reactions is recommended.
Moderate renal impairment: Dosage adjustment and enhanced monitoring are required.
Mild hepatic impairment: No dosage adjustment is required.
Moderate hepatic impairment: Dosage adjustment and close monitoring of adverse reactions are required.