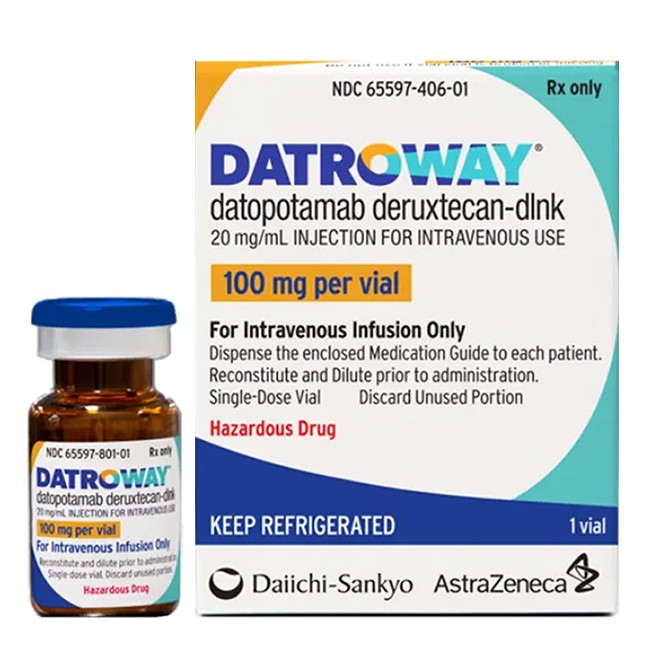
Derabrutinib (Datroway) is a Trop-2-targeted antibody-drug conjugate (ADC) indicated for the treatment of adult patients with unresectable or metastatic HR-positive, HER2-negative breast cancer who have received prior endocrine therapy and chemotherapy. As a prescription medication, the standardization of its procurement channels and the reliability of product authenticity directly affect treatment outcomes and patient safety.
What are the Procurement Channels for Derabrutinib (Datroway)?
Overseas Procurement
Patients may consult and purchase derabrutinib at hospital pharmacies or licensed drugstores in countries/regions where the medication is approved for marketing.
Due to potential price variations caused by regional differences, exchange rate fluctuations, and other factors, patients should conduct budget planning prior to purchase.
Procurement through Medical Service Institutions
Patients may consult domestic overseas medical service institutions collaborating with international pharmacies or pharmaceutical companies.
These institutions typically provide legal import channels and offer professional consultation and guidance.
Precautions for the Procurement and Use of Derabrutinib (Datroway)
Strict Adherence to Prescription and Dosage Specifications
The recommended dosage is 6 mg/kg (maximum dose of 540 mg for patients weighing ≥90 kg), administered as an intravenous infusion every 3 weeks until disease progression or unacceptable toxicity occurs.
If dosage interval adjustments are required, physicians should conduct dynamic assessments based on changes in patient weight and the severity of adverse reactions.
Contraindications and Special Population Warnings
Pregnant Women: The drug may cause embryo-fetal toxicity. Effective contraceptive measures must be used during treatment and for 7 months after the last dose.
Lactating Women: Breastfeeding is prohibited during treatment and for 1 month after discontinuation.
Patients with Hepatic or Renal Impairment: Enhanced monitoring of adverse reactions is required for patients with moderate to severe renal impairment; dosage infusion regimens should be adjusted for patients with moderate hepatic impairment.
Identification and Management of Severe Adverse Reactions
Interstitial Lung Disease (ILD)/Pneumonitis: Incidence rate of 4.2%, including 0.3% fatal cases. If symptoms such as cough, dyspnea, or fever occur, discontinue the drug immediately and initiate glucocorticoid therapy.
Ocular Adverse Reactions: 51% of patients experience symptoms such as dry eye or keratitis. It is recommended to use preservative-free lubricating eye drops daily and avoid wearing contact lenses.
How to Verify the Authenticity of Derabrutinib (Datroway)?
Packaging Integrity Verification
100 mg lyophilized powder in a single-dose vial.
Intact anti-counterfeiting seal.
Clear marketing authorization number and batch number.
Suspect counterfeit products if the packaging seal is broken, labels are illegibly printed, or batch numbers are worn.
Drug Characteristic and Reconstitution Verification
The lyophilized powder appears white to off-white; the reconstituted solution is clear, colorless to pale yellow.
Do not use if particles, turbidity, or abnormal discoloration is observed.
Information Tracing and Official Verification
Query production and circulation records through the national drug traceability platform.
Verify that the package insert content matches the official version, with particular attention to the manufacturer information (Daiichi Sankyo Co., Ltd.), indication scope, and dosage form/specification.