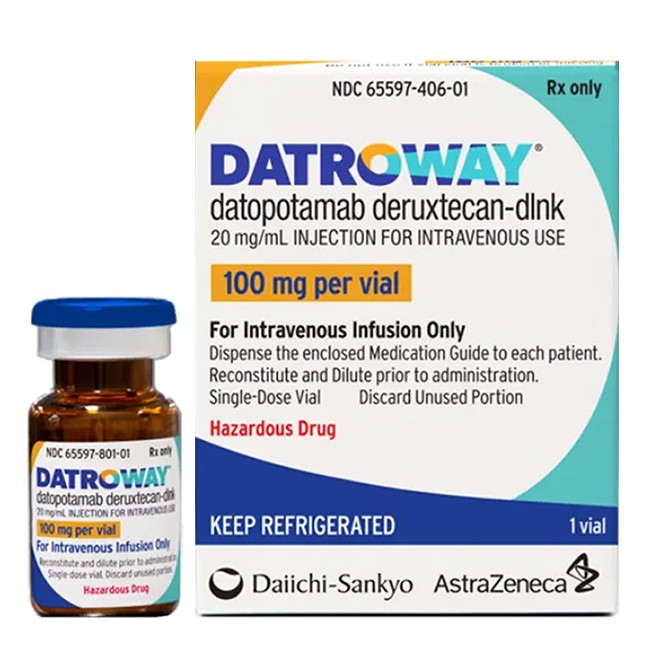
Datroway(DatopotamabDeruxtecan-dlnk) Instructions:Uses,Dosage, Side Effects
DATROWAY (datopotamab deruxtecan-dlnk) is a Trop-2-directed antibody-drug conjugate (ADC) that combines a humanized monoclonal antibody with a topoisomerase I inhibitor. The ADC is designed to deliver the cytotoxic agent directly to tumor cells expressing Trop-2, leading to DNA damage and cell death. It is supplied as a lyophilized powder in single-dose vials and is administered via intravenous infusion. This innovative therapy represents a targeted approach for treating advanced breast cancer in specific patient populations.


