Ivosidenib (Tibsovo) represents a pivotal breakthrough in the field of precision medicine. By targeting malignant tumors with specific genetic mutations, it offers a novel therapeutic option for patients.
What Are the Indications for Ivosidenib (Tibsovo)?
Newly Diagnosed Acute Myeloid Leukemia
Indicated for adult patients aged 75 years and older with newly diagnosed acute myeloid leukemia (AML), or patients ineligible for intensive induction chemotherapy due to comorbidities.
This drug can be administered either as monotherapy or in combination with azacitidine, providing a critical treatment pathway for elderly patients unsuitable for standard chemotherapy.
Relapsed or Refractory Acute Myeloid Leukemia
Specifically indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia, opening up new therapeutic avenues for patients who have failed conventional treatments.
Relapsed or Refractory Myelodysplastic Syndromes
Indicated for the treatment of adult patients with relapsed or refractory myelodysplastic syndromes (MDS), expanding the therapeutic landscape for hematological malignancies.
Locally Advanced or Metastatic Cholangiocarcinoma
Indicated for adult patients with locally advanced or metastatic cholangiocarcinoma who have received prior treatment, offering a targeted therapy option for this refractory tumor type.
Specifications and Characteristics of Ivosidenib (Tibsovo)
Specification Parameters
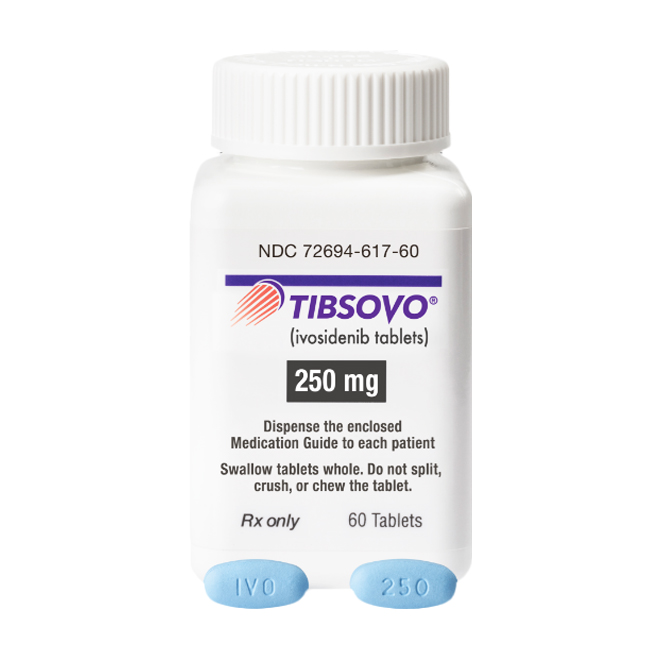
Ivosidenib is supplied as film-coated tablets, with a strength of 250 mg per tablet.
The tablets have a distinctive blue, oval, film-coated appearance, imprinted with "IVO" on one side and marked with "250" on the other for easy identification and verification.
Composition
In addition to the active ingredient ivosidenib, the tablets contain a variety of pharmaceutical excipients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate.
The film coating consists of FD&C Blue No. 2, hypromellose, lactose monohydrate, titanium dioxide, and triacetin, ensuring the stability and bioavailability of the drug.
Storage Conditions for Ivosidenib (Tibsovo)
Storage Temperature Requirements
Ivosidenib should be stored at room temperature between 20°C and 25°C (68°F and 77°F).
Short-term storage within the range of 15°C to 30°C (59°F to 86°F) is permissible.
Packaging Precautions
The drug is packaged in brown glass bottles containing 60 tablets, with a desiccant canister included in each bottle.
Important note for use: The desiccant must not be removed from the bottle to maintain the stability of the drug.