Tibsovo® (Ivosidenib) is an oral targeted therapy indicated for IDH1 mutations. It has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of specific types of acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and cholangiocarcinoma.
Channels for Purchasing Tibsovo® (Ivosidenib)
Overseas Purchase
Patients may consult and purchase the medication at hospital pharmacies or licensed drugstores in countries/regions where Tibsovo® has been officially launched.
Given that drug prices may be affected by regional differences, exchange rate fluctuations and other factors, patients are advised to make a detailed budget and plan in advance before purchasing.
Purchase via Medical Service Providers
Patients may consult domestic overseas medical service agencies that have partnerships with international pharmacies or pharmaceutical manufacturers.
These agencies typically offer legitimate import channels, along with professional consultation and guidance services.
Precautions for Purchasing Tibsovo® (Ivosidenib)
Genetic Testing
Complete genetic testing: Confirm the IDH1 mutation status through a qualified testing institution.
Obtain a professional prescription: A prescription must be issued by a hematologist or oncologist based on the test results and clinical assessment of the patient’s condition. Purchasing prescription drugs without a valid prescription is illegal and dangerous.
Verify Drug Information and Source
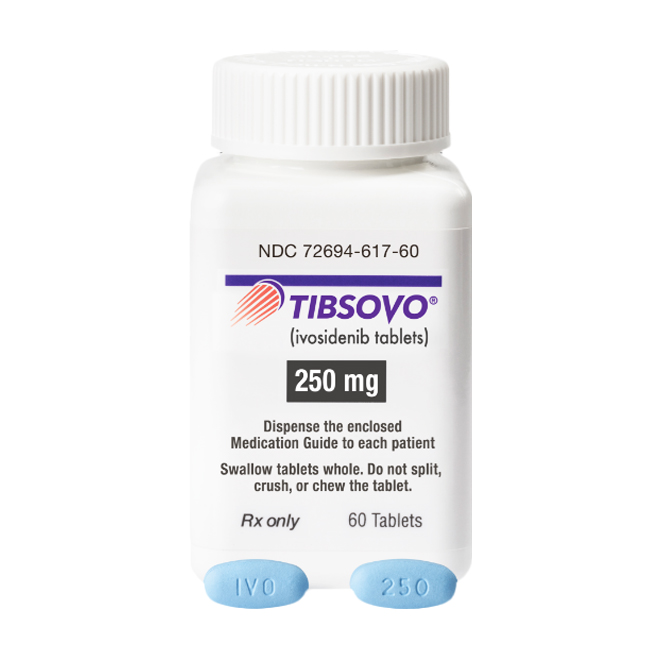
Outer packaging inspection: Confirm the accuracy of details including the brand name (TIBSOVO), active ingredient (Ivosidenib), dosage strength (e.g., 250 mg), and manufacturer (Servier Pharmaceuticals LLC).
Batch number and expiration date check: Ensure these details are clearly printed and verifiable through relevant official systems.
How to Distinguish Authentic Tibsovo® (Ivosidenib) from Counterfeits
Inspect Packaging and Tablet Appearance
Packaging quality: Authentic products feature high-quality printing, clear coloration, and standard packaging materials. Be wary of products with shoddy packaging, typographical errors, or ambiguous information.
Tablet appearance: The 250 mg strength of Ivosidenib is a blue, oval, film-coated tablet, engraved with “IVO” on one side and “250” on the other.
Carefully compare the tablet’s color, shape, and engravings against the official descriptions.
Check Traceability Information
Batch number verification: Legitimate drugs are labeled with a unique batch number and expiration date on the outer packaging.
Verification can be conducted through official channels of the partner pharmacies of the service provider or the pharmaceutical manufacturer (assistance from the service provider may be required).
Regulatory code verification: Drugs in some countries/regions are affixed with an official regulatory code, which can be verified by scanning the code via designated official websites or mobile applications.