Ivosidenib (Tibsovo) is a targeted therapy for IDH1-mutant acute myeloid leukemia, myelodysplastic syndromes, and cholangiocarcinoma. Its proper acquisition and standardized administration directly impact treatment efficacy and patient safety.
What Are the Purchase Channels for Ivosidenib (Tibsovo)?
Overseas Purchase
Patients may opt to consult and purchase the drug at hospital pharmacies or licensed drugstores in countries or regions where ivosidenib has been approved for marketing.
As drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase via Medical Service Institutions
Patients can consult domestic overseas medical service institutions that collaborate with international pharmacies or pharmaceutical enterprises.
These institutions can usually provide legal import channels, as well as professional consultation and guidance.
Precautions for Ivosidenib (Tibsovo)
Prescription-Only Purchase Requirement
Ivosidenib is a strictly controlled prescription drug, and it is strictly prohibited to purchase it without a valid prescription.
Before administration, a physician must assess the patient’s condition, confirm the IDH1 mutation status, and formulate an individualized treatment plan.
Verification of Drug Version and Packaging
When purchasing, it is necessary to check whether the drug is the original research product or a generic version.
The original research drug is manufactured by Servier Pharmaceuticals LLC (USA), and its packaging should clearly display the manufacturer information, batch number, and expiration date.
If a generic version is chosen, ensure that it is produced by a reputable pharmaceutical enterprise that has obtained international certification.
Understanding Drug Storage Conditions
Ivosidenib should be stored at room temperature between 20°C and 25°C, protected from moisture and direct sunlight.
Upon purchase, inspect the packaging for integrity and check whether a desiccant is included in the bottle. Reject the product if there is any damage or poor sealing.
How to Identify Authentic Ivosidenib (Tibsovo)?
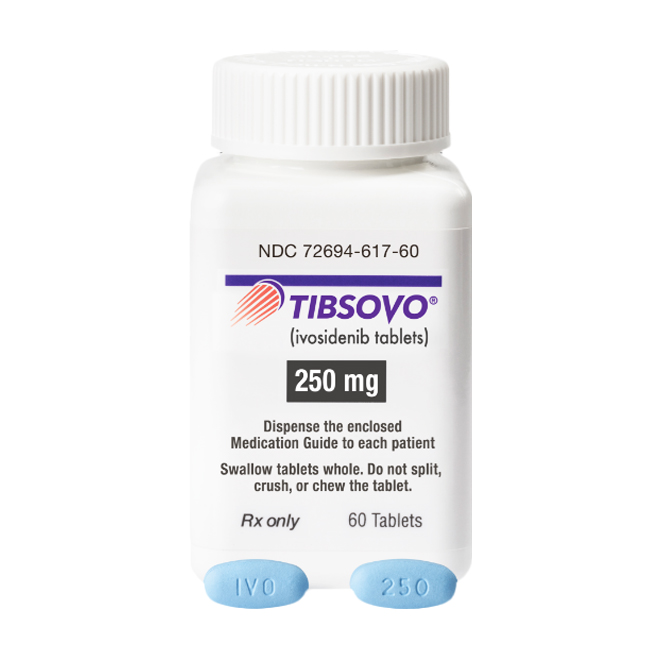
Observe the Physical Appearance of the Tablets
Authentic ivosidenib tablets are blue, oval-shaped, and film-coated, with "IVO" engraved on one side and "250" on the other.
Exercise high vigilance if the tablets show discrepancies in color, shape, or have blurred engravings.
Verify Packaging Information
Check the drug name and specification (250 mg per tablet).
Confirm the manufacturer (Servier Pharmaceuticals LLC).
Verify the approval number.
Check the production batch number and expiration date.
Review the storage conditions and administration instructions.
Monitor Medication Reactions
Authentic ivosidenib may cause some known adverse reactions after administration, such as changes in white blood cell count, fatigue, nausea, and QT interval prolongation.
If severe abnormal symptoms or those not mentioned in the package insert occur, seek medical attention promptly and verify the source of the drug.