Ivosidenib (Tibsovo) is an innovative isocitrate dehydrogenase-1 (IDH1) inhibitor, which represents a breakthrough in the treatment of hematological malignancies and solid tumors.
Dosage and Administration, Recommended Dose of Ivosidenib (Tibsovo)
Standard Administration Regimen
The recommended dose of ivosidenib is 500 mg orally once daily, to be continued until disease progression or unacceptable toxicity occurs.
The medication should be taken at the same fixed time each day.
It may be administered with or without food, but consumption of high-fat meals (e.g., meals containing 58 grams of fat) must be strictly avoided.
If vomiting occurs after dosing, no additional dose should be taken; the next scheduled dose should be taken as planned.
If a dose is missed, it should be taken as soon as the patient remembers, provided that at least 12 hours elapse before the next scheduled dose.
Dosage Form Characteristics and Administration Method
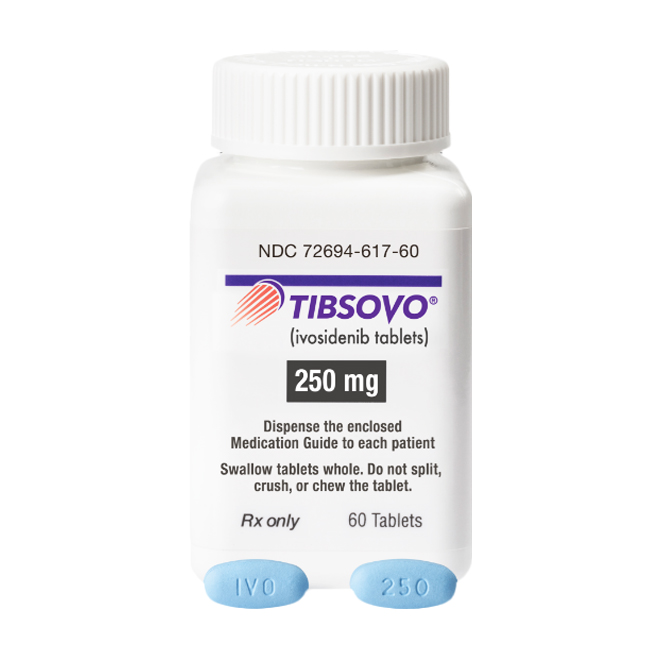
This product is available as 250 mg film-coated tablets, which are blue, oval-shaped, and imprinted with "IVO" and "250".
The tablets must be swallowed whole; they should not be split, crushed, or chewed, so as to ensure that the drug release characteristics are not affected.
Dose Adjustment of Ivosidenib (Tibsovo)
Management of Differentiation Syndrome
If symptoms such as fever, cough, dyspnea, rash, oliguria, hypotension, rapid weight gain, or edema of the extremities occur.
Dexamethasone (10 mg intravenous injection every 12 hours) should be administered immediately, along with hemodynamic monitoring.
If severe symptoms persist 48 hours after glucocorticoid treatment, ivosidenib should be withheld, and treatment may be resumed when symptoms improve to grade 2 or lower.
Management of Electrocardiogram QT Interval Prolongation
QTc > 480–500 milliseconds: Withhold the drug, monitor electrolytes, and resume treatment at the daily dose of 500 mg when QTc returns to ≤ 480 milliseconds.
QTc > 500 milliseconds: Withhold the drug, and resume treatment at the daily dose of 250 mg when QTc returns to within 30 milliseconds of the baseline value or ≤ 480 milliseconds.
If accompanied by life-threatening arrhythmia symptoms: Discontinue the drug permanently.
Management of Guillain-Barré Syndrome
Ivosidenib treatment should be permanently discontinued immediately upon confirmed diagnosis.
Administration in Special Populations of Ivosidenib (Tibsovo)
Patients with Hepatic or Renal Impairment
Mild or moderate hepatic impairment (Child-Pugh Class A or B): No initial dose adjustment is required.
Severe hepatic impairment (Child-Pugh Class C): Safety data are lacking; a thorough benefit-risk assessment is needed.
Mild or moderate renal impairment (eGFR ≥ 30 mL/min): No dose adjustment is required.
Severe renal impairment (eGFR < 30 mL/min) and patients requiring dialysis: It is recommended to conduct a full risk assessment before initiating treatment.
Women of Childbearing Potential and Lactating Women
Based on the results of animal embryotoxicity studies, ivosidenib may cause fetal harm.
Breastfeeding should be discontinued during treatment and for 1 month after the last dose.