Vabysmo (faricimab) is a dual inhibitor of vascular endothelial growth factor (VEGF) and angiopoietin-2 (Ang-2), widely used in the treatment of neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), and macular edema following retinal vein occlusion (RVO).
What Are the Purchase Channels for Vabysmo (Faricimab)?
Overseas Purchase
Patients may consult and purchase Vabysmo at hospital pharmacies or licensed drugstores in countries/regions where the medication is approved for marketing.
Due to potential price variations caused by regional differences, exchange rate fluctuations, and other factors, patients should make a budget and plan in advance before purchasing.
Purchase Through Medical Service Institutions
Patients may consult domestic overseas medical service institutions collaborating with international pharmacies or pharmaceutical companies.
These institutions typically provide legal import channels and offer professional consultation and guidance.
Precautions for Purchase and Use of Vabysmo (Faricimab)
Strictly Follow Prescriptions and Dosage Regimens
For nAMD patients: Administer 4 initial doses once every 4 weeks, then adjust the interval based on optical coherence tomography (OCT) and visual acuity assessment results (e.g., Weeks 28 and 44; Weeks 24/36/48; or Weeks 20/28/36/44).
For DME patients: Two options are available: (a) After at least 4 doses administered once every 4 weeks, adjust the interval based on central subfield thickness (CST); or (b) Administer 6 initial doses once every 4 weeks, then switch to once every 8 weeks.
For RVO patients: Recommended dosage is once every 4 weeks for 6 consecutive months.
Contraindications and Special Population Warnings
Absolute contraindications: Contraindicated in patients with ocular or periocular infections, active intraocular inflammation, or hypersensitivity to faricimab or any of its components.
Use in pregnant women may increase the risk of miscarriage; decisions should be made by physicians after evaluating the benefits and potential risks.
Lactating women should use the medication with caution.
Women of childbearing age must use effective contraception during treatment and for at least 3 months after the last dose.
Storage and Transportation Specifications
Unopened vials/syringes must be stored refrigerated at 2°C–8°C; freezing or shaking is prohibited.
Before use, the medication may be stored at room temperature (20°C–25°C) for no more than 24 hours, and must be kept in the original packaging protected from light.
Authenticity Verification of Vabysmo (Faricimab)
Packaging and Label Inspection
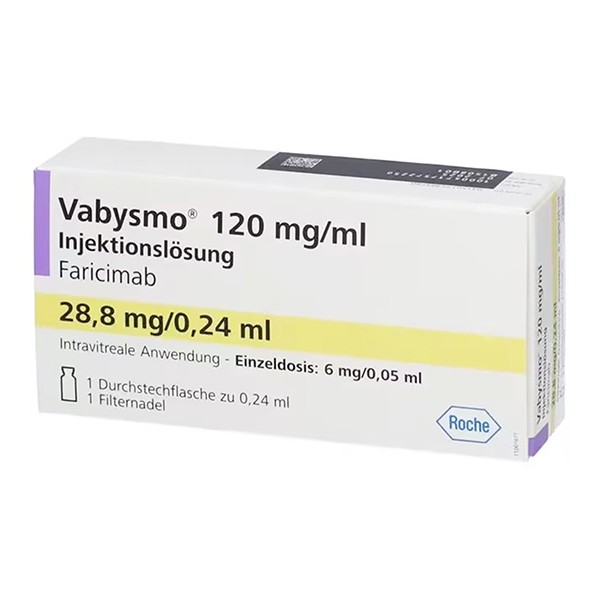
Authentic prefilled syringes include a sterile syringe in a sealed tray and an injection filter needle (30G × ½ inch) with an integrated filter. Vial kits contain a glass vial and a sterile 5-micron blunt transfer filter needle.
Check that the drug approval number (e.g., NDC 50242-096-06) is clear and complete, and that the packaging is free of damage or tampering.
Medication Appearance Confirmation
Vabysmo is a clear to opalescent, colorless to tan liquid.
Do not use if particles, turbidity, or abnormal discoloration is observed.
Traceability and Information Verification
Query the production batch number and circulation path through the official drug traceability platform.
Verify that the content of the package insert matches the official version, with special attention to the dosage form/specification (6mg/0.05mL) and manufacturer information (Genentech/Roche Group).