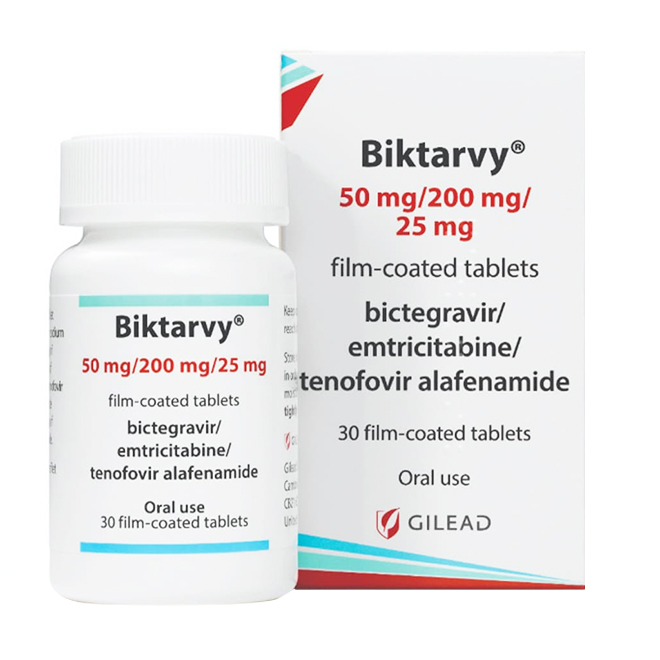
Biktarvy is a highly effective three-drug fixed-dose combination formulation specifically designed for the treatment of human immunodeficiency virus type 1 (HIV-1) infection.
What Are the Indications for Biktarvy (Biktarvy)?
Complete Treatment Regimen
As a complete treatment regimen, Biktarvy is approved for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 14 kg.
It is specifically indicated for the following situations: Treatment-naive patients (patients with no prior antiretroviral treatment history).
Treatment-experienced patients who have not achieved virologic suppression, and in whom substitutions associated with resistance to integrase strand transfer inhibitors (INSTIs), emtricitabine, or tenofovir have not been detected or are not suspected.
In virologically suppressed patients, Biktarvy may be used to replace their current stable antiretroviral regimen, provided that no known or suspected substitutions associated with resistance to bictegravir or tenofovir are present in the patients' virus.
This medication is indicated for virologically suppressed adult patients weighing at least 25 kg or those receiving chronic hemodialysis with a creatinine clearance rate below 15 mL/min.
Specifications and Properties of Biktarvy (Biktarvy)
Dosage Specifications
50 mg/200 mg/25 mg tablets: Each tablet contains 50 mg of bictegravir (equivalent to 52.5 mg of bictegravir sodium), 200 mg of emtricitabine, and 25 mg of tenofovir alafenamide.
These tablets are purplish-brown, capsule-shaped, film-coated, with "GSI" engraved on one side and "9883" on the other.
30 mg/120 mg/15 mg tablets: Each tablet contains 30 mg of bictegravir (equivalent to 31.5 mg of bictegravir sodium), 120 mg of emtricitabine, and 15 mg of tenofovir alafenamide (equivalent to 16.8 mg of tenofovir alafenamide fumarate).
These tablets are pink, capsule-shaped, film-coated, with "GSI" engraved on one side and "B" on the other; alternatively, one side has a non-functional score line and the other side is engraved with "BVY".
Physical Properties
All dosage forms of tablets adopt film-coating technology.
The coating materials include black iron oxide, red iron oxide, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Storage Methods for Biktarvy (Biktarvy)
Packaging and Storage
Bottle packaging: Each bottle contains 30 tablets, with a silica gel desiccant, polyester coil, and child-resistant cap.
Bottle-packaged medications should be stored sealed below 30°C (86°F). Do not remove the desiccant packet.
Blister packaging: Sealed with child-resistant aluminum foil composite lids, each blister contains 30 tablets.
The storage temperature is 25°C (77°F), with allowable fluctuations in the range of 15-30°C (59-86°F).