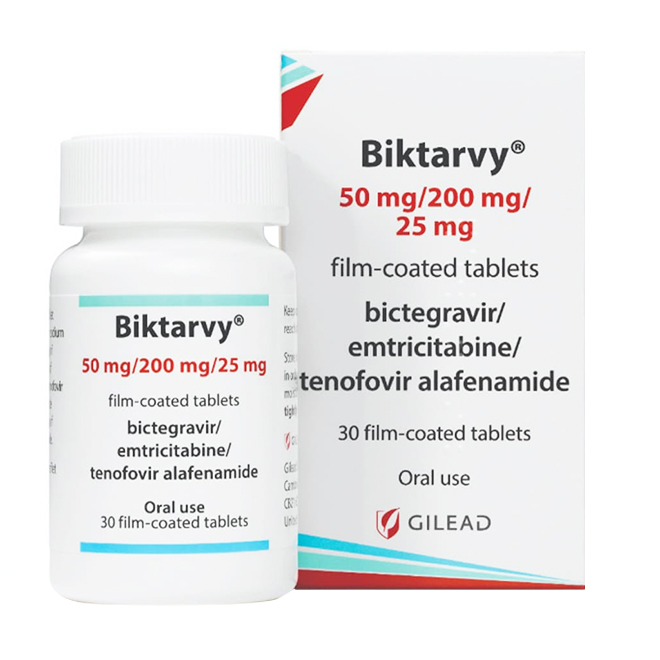
Biktarvy is a three-in-one fixed-dose combination formulation that has attracted significant attention due to its remarkable therapeutic efficacy. Standardized acquisition, safe administration, and authenticity verification of the medication are key steps to ensure optimal treatment outcomes.
What Are the Purchase Channels for Biktarvy (Biktarvy)?
Overseas Purchase
Patients can choose to consult and purchase Biktarvy at hospital pharmacies or licensed drugstores in countries or regions where the medication has been marketed.
Since drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase via Medical Service Institutions
Patients can consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions can usually provide legal import channels, as well as professional consultation and guidance.
Precautions for Purchasing Biktarvy (Biktarvy)
Professional Medical Evaluation First
A comprehensive medical assessment, including hepatitis B virus testing, must be conducted before obtaining Biktarvy.
For patients with concurrent hepatitis B infection, special vigilance is required regarding the risk of acute hepatitis exacerbation after discontinuation of the medication.
Comprehensive Renal Function Monitoring
Before initiating treatment, it is necessary to assess serum creatinine, estimated creatinine clearance rate, urine glucose, and urine protein.
In particular, patients with chronic kidney disease should also undergo serum phosphorus testing.
Medication Guidelines for Special Populations
Biktarvy is not recommended for patients with severe renal impairment.
This includes patients with an estimated creatinine clearance rate of 15 to less than 30 mL/min.
Patients with end-stage renal disease who are not receiving chronic hemodialysis.
As well as treatment-naive patients with end-stage renal disease receiving chronic hemodialysis.
Methods for Authenticity Verification of Biktarvy (Biktarvy)
Drug Packaging Identification
Biktarvy is available in two specifications: The 50mg/200mg/25mg tablet is purplish-brown, capsule-shaped, film-coated, engraved with "GSI" on one side and "9883" on the other.
The 30mg/120mg/15mg tablet is pink, capsule-shaped, film-coated, engraved with "GSI" on one side and "B" on the other; alternatively, it has a non-functional score line on one side and "BVY" engraved on the other.
Source Traceability System
The approval number, production batch number, and expiration date on the drug packaging can be checked with the manufacturer or official channels for verification.
The official website of the National Medical Products Administration provides a drug inquiry function.
Appearance Feature Verification
A preliminary judgment can be made based on the tablet's color, shape, engravings, and other characteristics.
Attention should be paid to the quality of packaging materials and printing details; authentic products feature sophisticated packaging craftsmanship and clear text.