Pirtobrutinib is a novel kinase inhibitor that received its first approval in the United States in 2023. As a non-covalent inhibitor of Bruton's Tyrosine Kinase (BTK), it demonstrates unique advantages in treating B-cell malignancies that have developed resistance to traditional BTK inhibitors.
What Are the Indications for Pirtobrutinib?
Mantle Cell Lymphoma (MCL)
This drug is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma.
These patients must have previously received at least two lines of systemic therapy, which must include one BTK inhibitor.
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL)
Pirtobrutinib is also indicated for the treatment of adult patients with chronic lymphocytic leukemia or small lymphocytic lymphoma.
These patients must have previously received at least two prior lines of therapy, which must include one BTK inhibitor and one BCL-2 inhibitor.
Dosage Forms, Strengths, and Recommended Dosage Regimen of Pirtobrutinib
Dosage Forms and Strengths
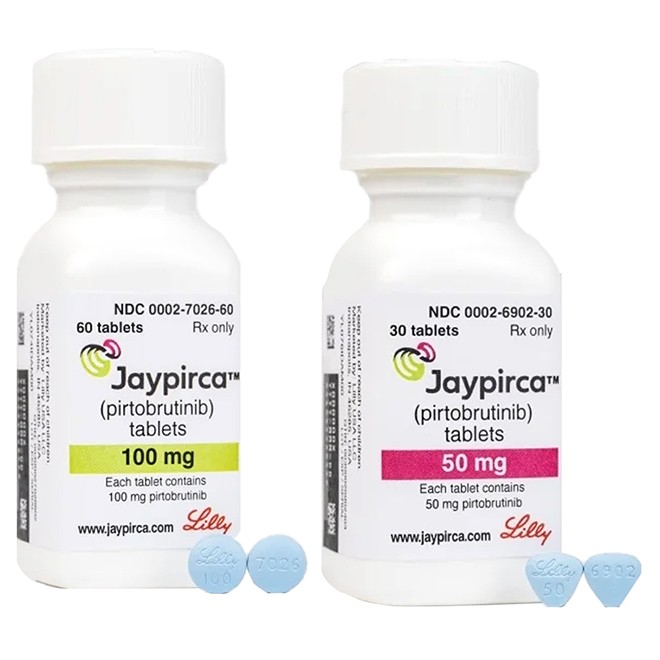
50 mg tablets: Blue, arc-shaped triangular, film-coated tablets. One side is engraved with "Lilly50" and the other side with "6902".
100 mg tablets: Blue, round, film-coated tablets. One side is engraved with "Lilly100" and the other side with "7026".
Recommended Dosage Regimen
The standard dosage is 200 mg taken orally once daily, until disease progression or unacceptable toxicity occurs.
The drug can be taken with or without food, but must be swallowed whole. It should not be split, crushed, or chewed, and any broken tablets should not be taken.
Storage Conditions for Pirtobrutinib
Storage Conditions
The medication should always be stored in its original packaging bottle.
The appropriate storage temperature is 20°C to 25°C (68°F to 77°F), with brief fluctuations allowed between 15°C and 30°C (59°F and 86°F).
Packaging and Dispensing Requirements
All packages are equipped with child-resistant caps.
50 mg tablets: Bottles containing 30 tablets, with NDC number 0002-6902-30.
100 mg tablets: Bottles containing 60 tablets, with NDC number 0002-7026-60.
The medication should be kept out of the reach of children to prevent accidental ingestion.
If a dose is missed and more than 12 hours have passed since the regular dosing time, the missed dose should not be made up. The next dose should be taken as scheduled.