Pirtobrutinib is a novel BTK kinase inhibitor. It provides a new treatment option for patients with relapsed or refractory mantle cell lymphoma (MCL) who have previously received at least two lines of systemic therapy (including a BTK inhibitor), as well as for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have received at least two lines of therapy (including a BTK inhibitor and a BCL-2 inhibitor).
What Are the Purchase Channels for Pirtobrutinib?
Overseas Purchase
Patients may choose to consult and purchase the medication at hospital pharmacies or licensed drugstores in countries or regions where pirtobrutinib has been approved for marketing.
Since drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase through Medical Service Institutions
Patients may consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions usually provide legal import channels and can offer professional consultation and guidance.
Precautions for Purchasing Pirtobrutinib
Medical Evaluation
A comprehensive medical evaluation must be completed before purchasing pirtobrutinib.
Doctors will assess the patient’s infection risk, bleeding tendency, cardiac function, and other indicators. These evaluation results will directly affect the formulation of the treatment plan and the adjustment of drug dosage.
Dosage Confirmation and Verification
The recommended dosage of pirtobrutinib is 200 mg taken orally once daily.
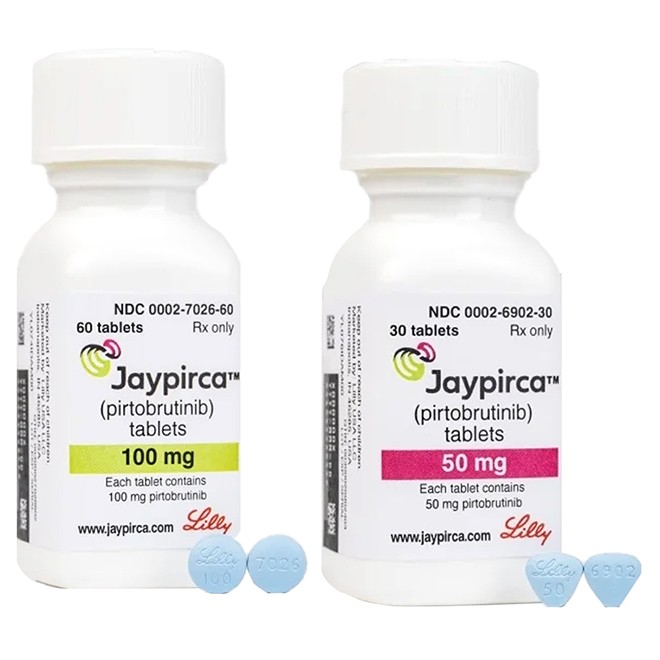
The medication is available in two strengths:
50 mg: blue, arc-shaped triangular film-coated tablets, engraved with "Lilly50" on one side and "6902" on the other.
100 mg: blue, round film-coated tablets, engraved with "Lilly100" on one side and "7026" on the other.
When purchasing, carefully check the drug strength to ensure the correct dosage 规格 is obtained.
Drug Interactions
Special attention must be paid to interactions with other medications.
Concomitant use with strong CYP3A inhibitors should be avoided, and concurrent use with strong or moderate CYP3A inducers should also be avoided.
How to Distinguish Genuine from Counterfeit Pirtobrutinib
Verification of Packaging Labels
Genuine pirtobrutinib is manufactured by Eli Lilly and Company, and the packaging should have clear brand logos.
Each bottle contains 30 tablets (for the 50 mg strength) or 60 tablets (for the 100 mg strength), and is packaged with a child-resistant cap.
Inspection of Physical Characteristics
Authentic tablets should be intact without cracks or damage.
According to the drug instructions, 50 mg tablets are blue, arc-shaped triangles, and 100 mg tablets are blue circles, with specific engravings clearly visible.
Drug Information Inquiry
Medications purchased through formal channels will provide complete drug traceability information.
Patients may request to check key information such as the drug’s approval number and production batch number.