Capivasertib is an AKT kinase inhibitor, providing a new treatment option for patients with HR-positive, HER2-negative advanced breast cancer. As a prescription medication, understanding its standardized purchase channels, mastering correct usage precautions, and learning to identify the authenticity of the drug are crucial for effective treatment.
What Are the Purchase Channels for Capivasertib?
Overseas Purchase
Patients may choose to consult and purchase Capivasertib at hospital pharmacies or legitimate drugstores in countries or regions where the drug has been launched.
Since drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make budget and plans in advance before purchasing.
Purchase through Medical Service Institutions
Patients can consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions usually provide legal import channels and can offer professional consultation and guidance.
Precautions for Purchasing Capivasertib
Prior Medical Evaluation
A comprehensive medical evaluation must be completed before purchasing Capivasertib.
According to the requirements of the drug package insert, fasting blood glucose and hemoglobin A1C levels need to be tested; these evaluation results will directly affect the formulation of the treatment plan and the adjustment of drug dosage.
Necessity of Genetic Testing
The use of Capivasertib is premised on confirming the presence of PIK3CA, AKT1, or PTEN gene mutations in tumor tissue through an FDA-approved detection method.
Dosage Confirmation and Verification
The recommended dosage of Capivasertib is 400 mg taken orally twice daily, for 4 consecutive days followed by 3 days off.
When purchasing, the drug specification should be carefully checked to ensure the correct dosage specification is obtained.
Authenticity Identification of Capivasertib
Verification of Packaging Markings
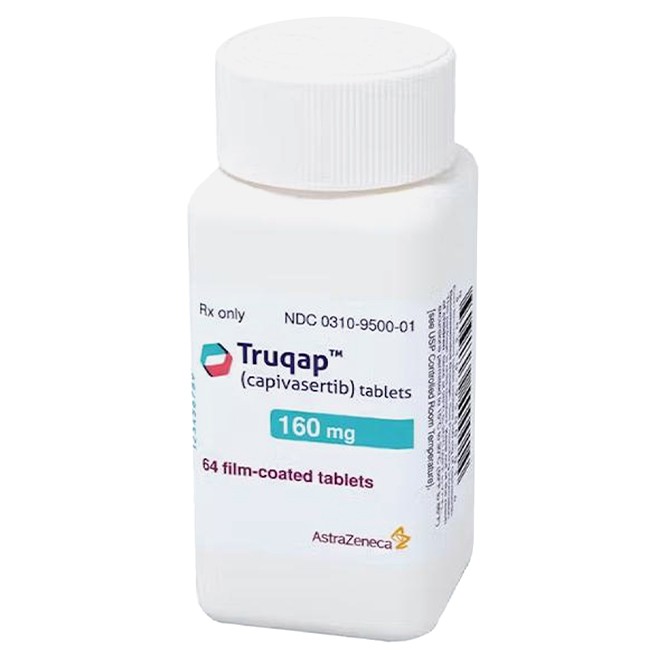
Genuine Capivasertib is manufactured by AstraZeneca, and the packaging should have a clear brand logo.
The drug is available in two specifications:
160 mg: Beige film-coated, round, biconvex tablets, engraved with "CAV" and "160" on one side.
200 mg: Beige film-coated, capsule-shaped, biconvex tablets, engraved with "CAV200" on one side.
Inspection of Physical Properties
Authentic tablets should be intact without cracks or damage.
According to the drug instructions, any broken, cracked, or incomplete tablets should not be taken.
Drug Information Inquiry
Through the official website of the National Medical Products Administration or relevant drug traceability platforms.
By entering the drug approval number and batch number, detailed drug information can be inquired, which is an important way to verify the authenticity of the drug.
Verification through Professional Channels
When purchasing, request the provision of complete drug instruction documents and traceability information.
Drugs from regular channels will be accompanied by detailed usage instructions and adverse reaction information.