Resmetirom (Rezdiffra) is the first drug approved by the U.S. FDA in 2024 for the treatment of non-cirrhotic non-alcoholic steatohepatitis (NASH). Its standardized acquisition and authenticity identification are of great importance for treatment.
How to Purchase Resmetirom (Rezdiffra)
Overseas Purchase
Patients may choose to consult and purchase Resmetirom at hospital pharmacies or authorized retail pharmacies in countries or regions where the drug has been launched.
Since drug prices may be affected by factors such as regional differences and exchange rate fluctuations, patients need to make a budget and plan in advance before purchasing.
Purchase via Medical Service Institutions
Patients may consult domestic overseas medical service institutions that cooperate with international pharmacies or pharmaceutical companies.
These institutions can usually provide legal import channels and offer professional consultation and guidance.
Precautions for Purchasing Resmetirom (Rezdiffra)
Strictly Follow Dosage Standards
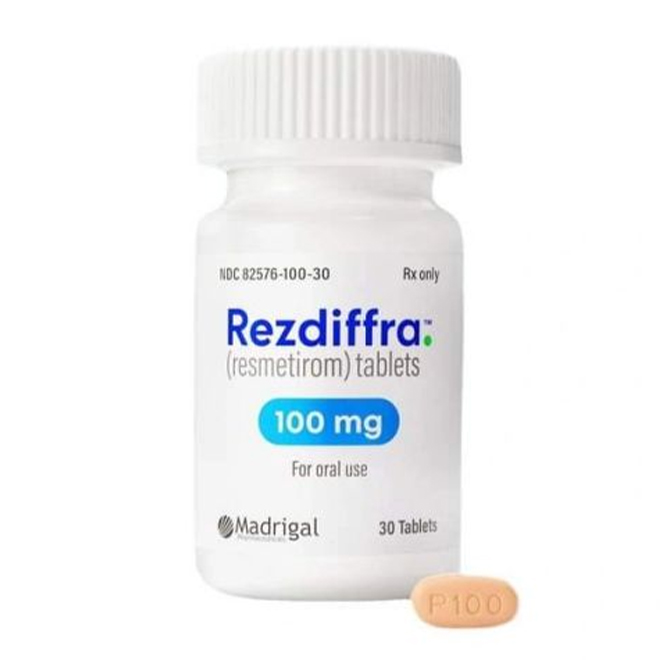
The drug is available in three specifications: 60 mg (white oval), 80 mg (yellow oval), and 100 mg (pinkish-beige oval).
Dosage must be strictly determined based on body weight:
Body weight < 100 kg: 80 mg per day.
Body weight ≥ 100 kg: 100 mg per day.
Precautions for Medication Use in Special Populations
Pregnant women: Clinical data are insufficient; the benefits and risks must be weighed.
Elderly patients: The incidence of adverse reactions may be higher.
Patients with liver impairment: No dosage adjustment is required for mild liver impairment (Child-Pugh Class A).
Screening for Contraindicated Drugs
Strong CYP2C8 inhibitors (e.g., gemfibrozil): Concurrent use is prohibited.
Moderate CYP2C8 inhibitors (e.g., clopidogrel): The dosage of resmetirom needs to be adjusted to 60 mg (for patients < 100 kg) or 80 mg (for patients ≥ 100 kg).
Statin drugs (e.g., atorvastatin, pravastatin): The daily dosage must be limited, and close monitoring for adverse reactions is required.
How to Identify the Authenticity of Resmetirom (Rezdiffra)
Verify Packaging Characteristics
Authentic products are packaged in white high-density polyethylene bottles, equipped with child-resistant caps and induction seals.
Check the completeness of information on the bottle label.
Verify the standardization of the drug approval number.
Confirm the accuracy of the manufacturer’s information.
Confirm Tablet Markings
60 mg tablets: White oval, engraved with "P60".
80 mg tablets: Yellow oval, engraved with "P80".
100 mg tablets: Pinkish-beige oval, engraved with "P100".
Verify via Official Channels
Check the drug supervision code.
Query authenticity through the official website of the pharmaceutical company.
Verify using the national drug supervision platform.