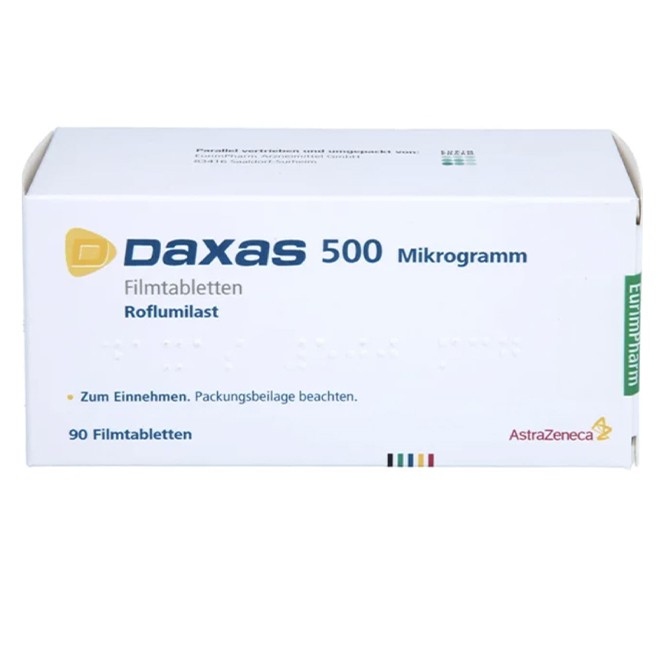
Roflumilast Tablets (Daxas) is a selective phosphodiesterase 4 (PDE4) inhibitor, primarily indicated for reducing the risk of acute exacerbations in patients with severe chronic obstructive pulmonary disease (COPD) complicated by chronic bronchitis and a history of acute exacerbations.
What Are the Purchase Channels for Roflumilast Tablets (Daxas)?
Overseas Purchase
Patients may opt to consult and purchase the medication at hospital pharmacies or licensed drugstores in countries/regions where roflumilast tablets have been approved for marketing.
Given that drug prices may be affected by factors such as regional variations and exchange rate fluctuations, patients are advised to make a sound budget and plan prior to purchase.
Purchase via Medical Service Institutions
Patients can consult domestic overseas medical service institutions that have established partnerships with international pharmacies or pharmaceutical manufacturers.
These institutions are generally able to provide legal import channels, as well as professional consultation and guidance services.
Precautions for Purchasing and Using Roflumilast Tablets (Daxas)
Strictly Comply with Prescription Requirements
Roflumilast is a strictly prescription-only medication and must never be purchased or administered without a valid prescription.
It shall be prescribed exclusively by respiratory specialists after a comprehensive assessment of the patient’s specific condition, including the severity of COPD, presence of chronic bronchitis, and history of acute exacerbations.
Hepatic Impairment Considerations
Contraindicated in patients with moderate to severe hepatic impairment (Child-Pugh Class B or C).
For patients with mild hepatic impairment, the medication may be administered only after a rigorous risk-benefit assessment by the attending physician.
Mental Health Considerations
This drug may induce or exacerbate psychiatric adverse reactions such as insomnia, anxiety, depression, and suicidal ideation.
For patients with a history of depression or suicidal tendencies, physicians must exercise extreme caution when evaluating the risks and benefits of treatment.
Patients and their family members should closely monitor changes in mood and behavior, and seek immediate medical attention if any abnormalities are observed.
Pay Attention to Drug Interactions
Avoid concomitant use: Do not co-administer with potent cytochrome P450 enzyme inducers (e.g., rifampicin, phenobarbital, carbamazepine, phenytoin), as this may significantly reduce the therapeutic efficacy of roflumilast.
Use with caution: Concomitant use with CYP3A4 or CYP1A2 inhibitors (e.g., erythromycin, ketoconazole, fluvoxamine) may increase plasma drug concentrations and the risk of adverse reactions, requiring careful evaluation by the physician.
Always inform your physician of all medications you are taking during consultations, including prescription drugs, over-the-counter medicines, herbal remedies, and dietary supplements.
How to Identify the Authenticity of Roflumilast Tablets (Daxas)?
Examine the Outer Packaging and Labeling
Official Markings: Verify the presence of clear manufacturer information (AstraZeneca), brand name (Daxas or Daliresp), generic name (Roflumilast), specification (250 mcg or 500 mcg), batch number, and expiration date on the packaging.
Anti-counterfeiting Features: Legitimate pharmaceutical packaging typically features elaborate design, high-definition printing, and specific anti-counterfeiting labels, laser holograms, or barcodes. You may scan the barcode to verify the product information.
Verify the Tablet Appearance
250 mcg tablets: Engraved with "D" on one side and "250" on the other side.
500 mcg tablets: Engraved with "D" on one side and "500" on the other side.
Carefully inspect the tablet’s color, size, and the clarity and regularity of the engravings. Counterfeit drugs often exhibit flaws such as uneven color, poor texture, or blurred, imprecise markings.